This information is presented as a theoretical framework intended for educational and introspective purposes only. It is not designed for entertainment, nor should it be construed as medical advice or guidance. The concepts discussed herein are not meant to replace professional medical consultation, diagnosis, or treatment.
Part 1 – Introduction.
This article presents a conceptual theory suggesting that lipopolysaccharides (LPS) and biofilm formation are foundational factors in many health issues, such as tumors, arthritis, gut disorders (including SIBO and leaky gut), autoimmune diseases, type 1 diabetes, liver fatty plaque overgrowth, and blood plaque. The idea is that LPS and biofilms serve as initial protective layers for bacteria against the body’s defenses, allowing them to persist and contribute to various conditions.

In this theory, bacteria, particularly Gram-negative types like E. coli and Fusobacterium nucleatum, produce LPS as a defensive mechanism. LPS serves as an “umbrella,” shielding these bacteria in less hostile environments like the gut when stomach acid is weakened due to high-carb diets. This weakening of stomach acid can occur with a pH shift to 5-7, making it easier for bacteria to colonize and form biofilms. Over time, these biofilms can develop into more complex structures known as the extracellular matrix, which provides a robust and protective environment for bacterial communities.
The hypothesis posits that these bacterial colonies and their protective structures may be implicated in various diseases. For instance, the presence of Fusobacterium nucleatum around colorectal tumors, typically found in the mouth, suggests a possible link between bacterial biofilms and cancer. Additionally, the formation of these protective barriers by bacteria can be seen as a critical factor in other health issues, where the body’s immune response may be insufficient to overcome these defenses.
This article encourages further research and exploration of the role of LPS and biofilms in disease pathology, while also highlighting the need for innovative treatments to address these bacterial structures. The use of binding agents like zeolite is proposed as one potential approach to supporting the body’s natural detoxification processes.
Disclaimer: This theory is presented for educational and introspective purposes only and should not be considered medical advice. It serves as a conceptual starting point for further scientific exploration and validation.
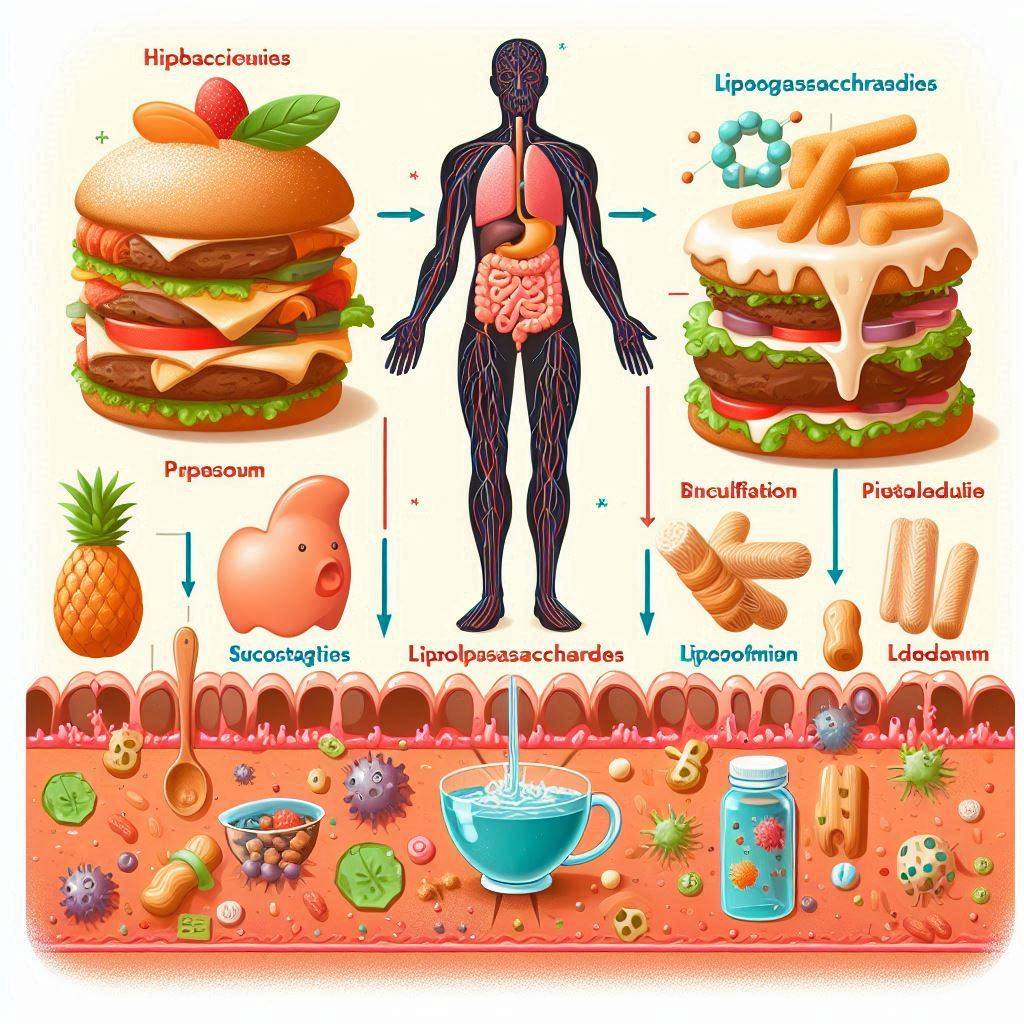
Part 2: It begins by your diet of high mono saccharides – aka the process carbs that are not bounded with fibers.
Over time, if an individual is not mindful of their diet, particularly the balance of fats and the production of ketone bodies, lipopolysaccharides (LPS) can proliferate in the body. This is especially true if the immune system is not fully aware or equipped to address these bacterial defenses. LPS, produced by Gram-negative bacteria, contribute to the formation of biofilms, which can develop into a robust extracellular matrix (ECM). This ECM acts as a fortified structure that protects bacterial colonies.
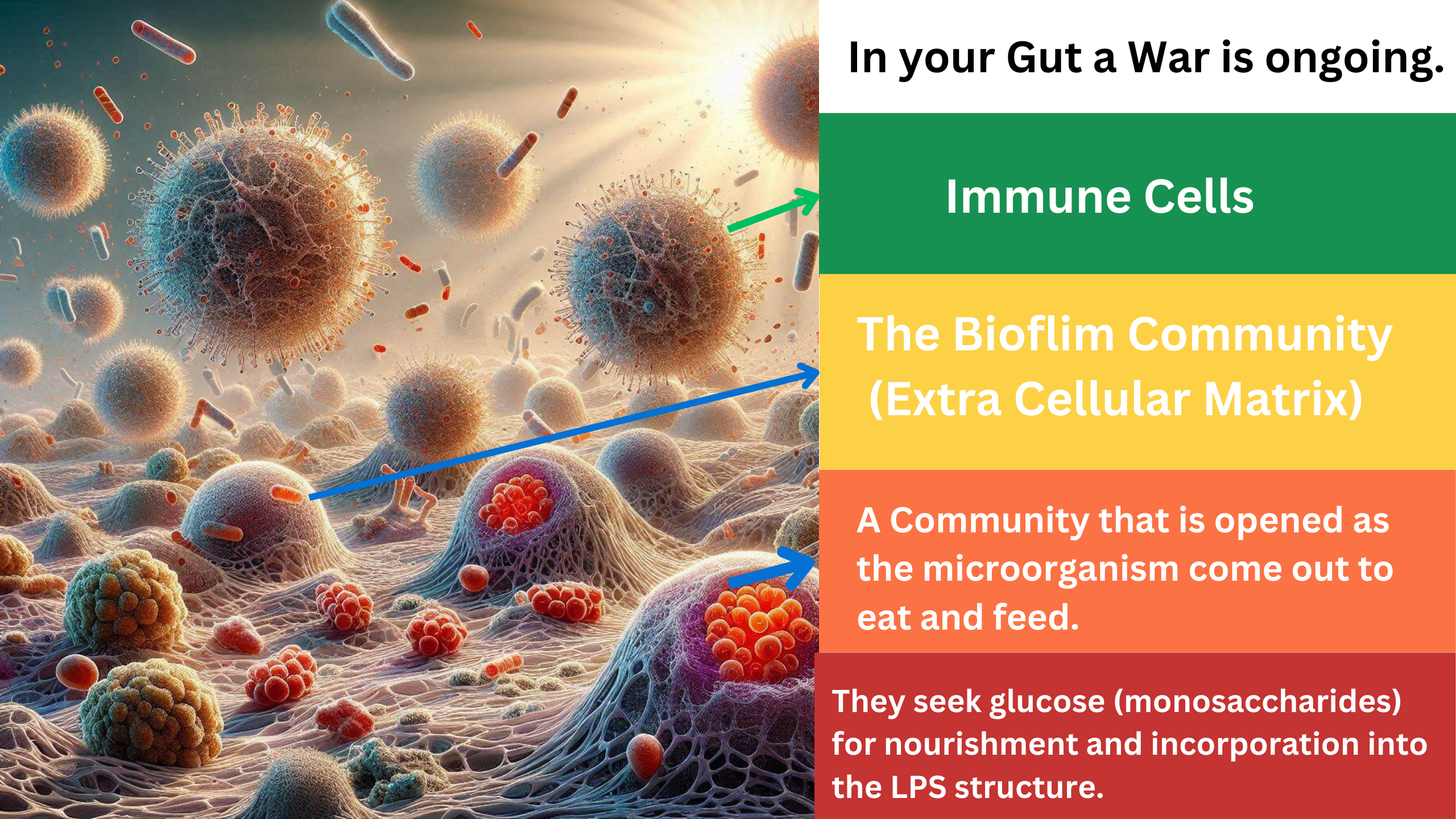
When the immune system detects these foreign invaders, it initially responds with a modest force, analogous to a few immune “hunters.” However, if these initial efforts are insufficient, the immune system escalates its response, sending stronger immune cells and mechanisms. This includes the activation of heavy artillery, such as macrophages and other immune cells, that attempt to attack and dismantle the bacterial biofilms and LPS structures.
Despite these efforts, the bacteria remain protected within their fortified ECM. The immune response, no matter how intense, often struggles to penetrate this barrier. The biofilm’s ECM effectively acts as a shield, deflecting the immune system’s attacks. The bacteria inside may suffer some casualties, but the core of the colony remains intact, protected by this resilient barrier. This can lead to a persistent state of inflammation as the immune system continues to target the invaders without being able to fully eradicate them.
This scenario underscores the challenge the immune system faces in dealing with biofilm-associated infections. The persistence of these biofilms can lead to chronic inflammation and contribute to various health conditions. Effective strategies to disrupt biofilms and the ECM, along with supporting the immune system, are crucial in managing these bacterial communities.
Part 3: Immune System and it ongoing response ( Auto Immune starts)
The immune system, feeling disheartened by its inability to penetrate the fortified defenses of the bacterial biofilms, questions its own effectiveness. The immune cells rally, preparing for another assault on the seemingly impenetrable extracellular matrix (ECM) protecting the bacteria, fungi, and other microbes. They launch a renewed attack with more intensity, using every weapon in their arsenal. Despite their best efforts, the microbes remain secure within their fortified community, shielded by the robust ECM they have constructed. The bacteria, in particular, are grateful to their human host for the continuous supply of carbohydrates, which fuels their growth and strengthens their defenses.
As the human host consumes more refined carbohydrates—such as bread, rice, flour, and sugars—the bacteria flourish. Occasionally, some bacteria venture outside their protective ECM to access the abundant glucose. These exposed bacteria become targets for the immune system, which quickly eliminates them. However, this intense immune response also damages the gut lining, creating cracks that can lead to ulcers. The immune system’s aggressive efforts inadvertently harm the host, causing inflammation and damage to the gut wall.
The cycle continues, with the host’s dietary choices feeding the bacterial community, allowing it to grow stronger and more resistant. The host begins to experience symptoms of illness, such as discomfort and digestive issues. Seeking relief, the host turns to herbal teas and eventually antibiotics. The introduction of antibiotics acts like a powerful bomb, wiping out many bacteria and pathogens outside the protective ECM. This gives the immune system a brief respite and the impression of victory.
However, the resilient microbes within the ECM remain largely unaffected, surviving the onslaught and waiting for the next opportunity to exploit the weakened state of the host. This scenario highlights the complex and often counterproductive battle between the body’s immune defenses and persistent microbial communities, particularly in environments like the gut where dietary and environmental factors play significant roles. The cycle of inflammation, immune response, and microbial adaptation underscores the importance of maintaining a balanced diet and gut health to support the body’s defenses.
Part 4: We need to migrate and venture to the other worlds.
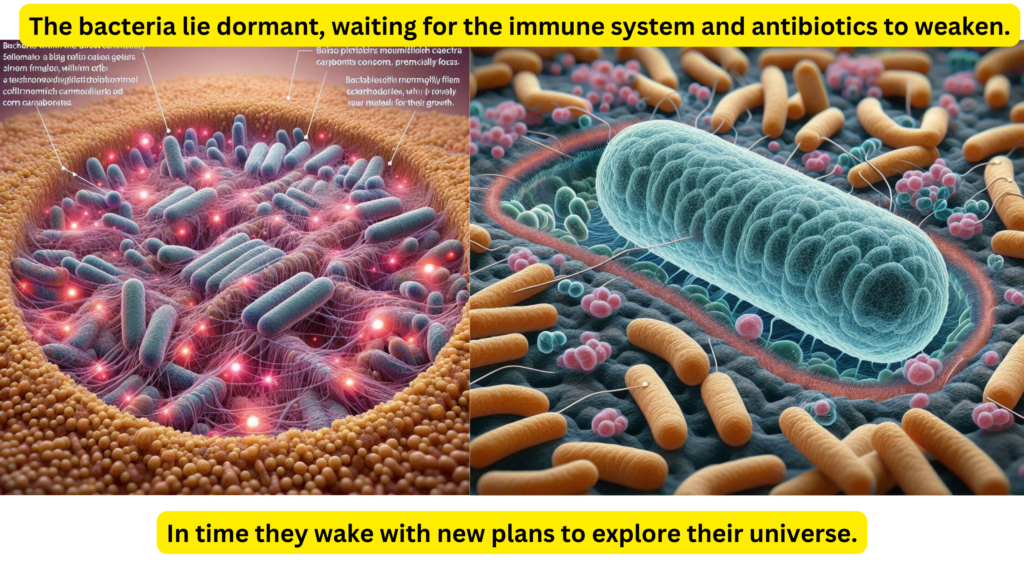
As the bacteria within the biofilm community center lie in wait, they bide their time, shielded by their extracellular matrix (ECM). They wait for signals that will prompt their host to consume more food, particularly carbohydrates, which provide the raw materials for their growth. When the host indulges in a high-carb meal, the bacteria quickly begin to replicate. Each new clone carries the genetic blueprint for survival, including the production of LPS and the formation of biofilm layers, further strengthening the ECM.
Despite the recent threat of antibiotics, which decimated many of their kind outside the protective ECM, the bacteria adapt and evolve. They use the nutrients provided by the host’s diet to reinforce their defenses, weaving LPS into biofilm, which then forms a more robust ECM. In a strategic move, some bacteria decide to expand their territory, migrating from the gut to the duodenum and into the small intestine. This migration is part of a survival strategy to ensure the continuation of their species even if the primary colony faces destruction.
As these bacteria establish themselves in new locations, they continue to provoke the immune system. The immune response, targeting these new colonies, causes damage to the duodenal and intestinal tissues, leading to ulceration. The destruction of these bacterial colonies releases LPS fragments into the digestive tract. These fragments are then processed by bile acids, breaking down into lipoproteins and fatty acids, which can be absorbed by the intestinal villi and enter the hepatic portal vein. This allows these bacterial components to enter the body, potentially causing systemic effects.
The bacteria’s strategy, leveraging the immune system’s response and the host’s dietary habits, demonstrates their adaptability and resilience. They manipulate the host environment to ensure their survival, even amidst the threat of antibiotics and immune responses. This narrative highlights the complex interplay between host, pathogen, and immune responses, emphasizing the importance of diet and immune system health in managing microbial populations within the body.