https://www.britannica.com
https://www.britannica.com/science/photosynthesis#/media/1/458172/161584
So the little plant cell is showing the process of the how glucose is made by plants – Actually a reverse process of the Human process more like they take C02 in the present of light and by product is Water – ATP.
The Water is not split to create the 02 and What (H2) gas – i wondering what the plants doing with the H2 gas?
Notice how the 1.35 says the WATER molecules are split, but does not show what happens to the H of the from the H20 – they make it seem that we getting a 02 from the process but I always thought waster was hydrogen 2 Oxygen.
Point here is that plants is sing this first day light process to create ATP – the same ATP that powers our Human bodies. They have to create ATP to do their work in the night. So plants are making glucose in the night, which signals the YING and YAN for humans not to take so much GLUCOSE at night, we must balance. Day Human and Animals should consume glucose and night avoid glucose. Let the plants make glucose at night.
At night it seem that the plant is pulling into it system the C02 in the presence of H20 to create glucose molecules, because it created the ATP to powerup the factories that can combine H20 and C02 to make glucose.
So solution for men in 2024 is to just combine H20 and Co2 and make glucose -there we have no Co2 problem anymore. Ok.
The conversion of atmospheric nitrogen into amino acids and subsequently into animal tissues is a complex, multi-step process involving various biological and chemical interactions. This process is vital as nitrogen, although abundant in the atmosphere, is not directly usable by most organisms. It must first be converted into more reactive forms through the nitrogen cycle. Here’s a comprehensive workflow of how atmospheric nitrogen is converted into amino acids and then integrated into animals:
Step 1: Nitrogen Fixation
Process: Atmospheric nitrogen (N₂)- about 78% of the atmosphere is converted into ammonia (NH₃) by nitrogen-fixing bacteria. These bacteria live freely in the soil or in symbiotic relationships with the roots of certain plants (e.g., legumes).
Environments:
- Symbiotic bacteria (e.g., Rhizobia in legume root nodules).
- Free-living bacteria (e.g., Azotobacter).
- Industrial processes (e.g., Haber-Bosch process, primarily for agricultural fertilizers).
Step 2: Nitrification
Process: Ammonia is further processed by nitrifying bacteria in the soil to form nitrites (NO₂⁻) and then nitrates (NO₃⁻). This process involves two main groups of bacteria:
- Ammonia-oxidizing bacteria (AOB) convert ammonia to nitrite.
- Nitrite-oxidizing bacteria (NOB) convert nitrite to nitrate.
Environments:
- Soil.
- Aquatic systems.
Step 3: Assimilation
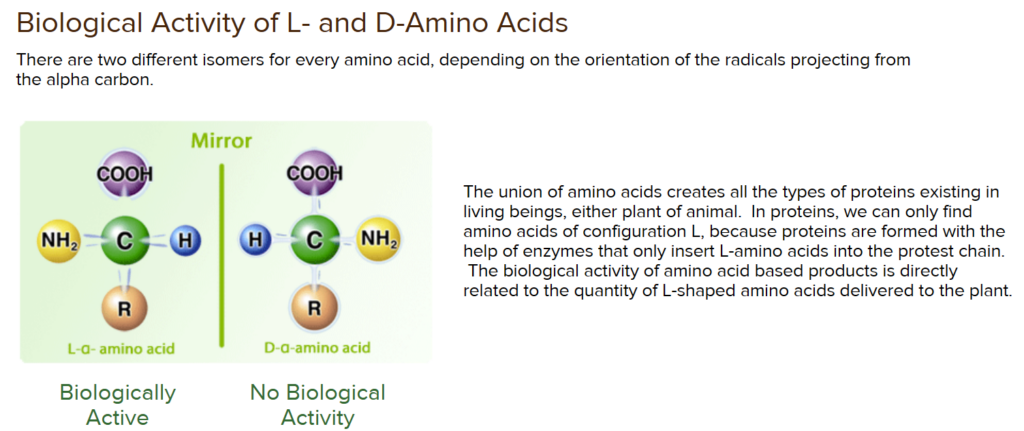
Process: Plants absorb nitrates from the soil through their roots. Once inside the plant, nitrates are converted into organic nitrogen forms, such as amino acids ( the plant form of amino acids are different to human), which are the building blocks of plant protein. ( Not yet amino acids that can be used by the animal)-the plant has to make the L- format of the same amino acids so that the animal can get the bioavailable amino acids. (So Enzymes in the plant knowns)
Environments:
- Terrestrial plant systems.
- Aquatic plant systems (e.g., algae).
Step 4: Consumption
Process: Animals obtain nitrogen by consuming plants or other animals. Herbivores eat plants to obtain proteins and amino acids, while carnivores and omnivores may consume other animals.
Environments:
- Various ecosystems including terrestrial and aquatic.
Step 5: Amino Acid Biosynthesis in Animals
Process: Once ingested, plant proteins are broken down into amino acids in the digestive system of animals. These amino acids are then used to synthesize new proteins needed for the animal’s body.
Environments:
- Digestive systems of animals.
Step 6: Trophic Transfer
Process: Nitrogen compounds (primarily in the form of amino acids and proteins) are transferred up the food chain through predation. Each trophic level gains nitrogenous compounds from the level below.
Environments:
- Food webs across ecosystems.
Step 7: Excretion and Decomposition
Process: Animals excrete waste nitrogen in the form of urea or other compounds, which are then converted back into ammonia by decomposers in the environment (e.g., bacteria and fungi). Similarly, when plants and animals die, decomposers break down their bodies, releasing nitrogen back into the soil in the form of ammonia, completing the nitrogen cycle.
Environments:
- Terrestrial and aquatic ecosystems.
Step 8: Denitrification
Process: Denitrifying bacteria convert excess nitrates in the soil back into nitrogen gas (N₂), which is released back into the atmosphere, completing the global nitrogen cycle.
Environments:
- Anoxic conditions in soils and sediments.
This workflow details the crucial roles of various microorganisms and environmental processes in converting inert atmospheric nitrogen into forms usable by living organisms, highlighting the complexity and interconnectedness of ecosystems.